A thermally isolated sample of an ideal gas at a fixed temperature is confined to one half of a container by an impermeable membrane.The other half of the container is evacuated.The membrane is then pierced and the gas is allowed to expand freely and to double its volume as shown.Which one of the following statements is true concerning this situation? 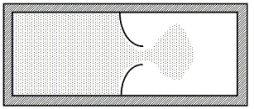
A) The process is reversible.
B) This is an isothermal process.
C) The entropy of the gas decreases.
D) The internal energy of the gas must decrease.
E) The temperature of the gas decreases to one-half of its original value.
Correct Answer:
Verified
Q1: If one complete cycle of a reversible
Q3: A container is divided into two chambers
Q4: A system containing an ideal gas at
Q5: Neon is a monatomic gas with a
Q6: 5.00 kg of liquid water is heated
Q7: Which one of the following pressure-volume graphs
Q8: When the gas enclosed beneath the piston
Q9: Enclosed beneath the moveable piston in the
Q10: Which one of the following situations is
Q11: An ideal gas absorbs 750 J of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents