Which of the following represents the equation for a first-order half-life?
A) t 1/2 = 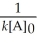
B) t 1/2 = 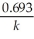
C) t 1/2 = 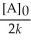
D) t 1/2 = 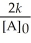
E) t 1/2 = 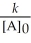
Correct Answer:
Verified
Q56: What data should be plotted to show
Q57: What data should be plotted to show
Q57: Given the following rate law, how does
Q58: Which of the following represents the equation
Q62: Derive an expression for a "1/3-life" for
Q66: The first-order decomposition of N2O at 1000
Q69: The rate constant for a second-order reaction
Q76: Which of the following statements is FALSE?
A)
Q77: If the concentration of a reactant is
Q87: For a reaction, what generally happens if
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents