Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements: 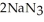 (s) → 2Na(s) +
(s) → 2Na(s) + 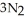 (g) How many moles of
(g) How many moles of 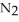 are produced by the decomposition of 1.25 mol of sodium azide?
are produced by the decomposition of 1.25 mol of sodium azide?
A) 0.833
B) 3.75
C) 1.88
D) 0.417
E) 0.625
Correct Answer:
Verified
Q1: According to the following reaction,what amount of
Q39: Choose the reaction that represents the combustion
Q40: How many moles of PCl5 will be
Q41: How many moles of BCl3 are needed
Q42: According to the following balanced reaction,how many
Q45: Carbonic acid can form water and carbon
Q46: How many molecules of HCl are formed
Q47: Balance the chemical equation given below,and determine
Q48: Magnesium burns in air with a dazzling
Q107: Dinitrogen monoxide gas decomposes to form nitrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents