The specific heat capacity of liquid water is 4.18 J/g-K.How many joules of heat are needed to raise the temperature of 5.00 g of water from 25.1°C to 65.3°C?
A) 48.1 J
B) 840 J
C) 1.89 × 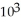 J
J
D) 2.08 × 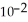 J
J
E) 54.4 J
Correct Answer:
Verified
Q95: A sample of copper absorbs 43.6 kJ
Q96: Give the units of heat capacity.
A) J/°C
B)
Q97: Give the units of specific heat capacity.
A)
Q99: What is the enthalpy change (in kJ)of
Q101: Which of the following processes is endothermic?
A)
Q102: Using the following equation for the combustion
Q103: At 1 atm pressure,the heat of sublimation
Q104: Which of the following processes is exothermic?
A)
Q113: For a particular process that is carried
Q134: The specific heat of copper is 0.385
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents