Consider the following image. 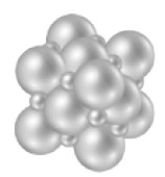 What type of solid is represented by this particulate-level model?
What type of solid is represented by this particulate-level model?
A) amorphous solid
B) molecular crystal
C) ionic crystal
D) covalent network solid
E) metallic crystal
Correct Answer:
Verified
Q18: If substance X has stronger intermolecular attractions
Q19: The structural formula for glycerin is shown
Q20: The kinetic molecular theory as applied to
Q21: A sample of solid silver releases 476
Q22: Calculate the heat of fusion of an
Q24: Among the following,identify the correct statement about
Q25: A substance that melts at 122°C is
Q26: The main reason equilibrium vapor pressure is
Q27: The heat of fusion of gold is
Q28: The specific heat of solid gold is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents