The structural formula for glycerin is shown below.Compare the intermolecular forces in glycerin with those in n-hexane,C6H14,in which all carbons are in a continuous chain.Which of the following statements is true? 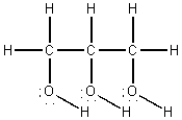
A) The principal intermolecular forces in glycerin are induced dipole forces; in hexane, hydrogen bonds
B) Hydrogen bonding is present in glycerin, but not in hexane
C) Hydrogen bonding is present in both compounds
D) Both compounds exhibit dipole-dipole forces
E) Induced dipole forces are present in neither compound
Correct Answer:
Verified
Q14: Select from the following the statement that
Q15: Draw the Lewis diagrams of CH3OH and
Q16: Considering the molecular mass and polarity influences
Q17: Acetone,a highly volatile liquid,is placed in a
Q18: If substance X has stronger intermolecular attractions
Q20: The kinetic molecular theory as applied to
Q21: A sample of solid silver releases 476
Q22: Calculate the heat of fusion of an
Q23: Consider the following image. Q24: Among the following,identify the correct statement about![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents