What is the pressure of a 1.0 L flask containing 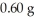 of He at 25°C? (R= 0.0821 L atm/ mol K)
of He at 25°C? (R= 0.0821 L atm/ mol K)
A) 98 atm
B) 3.7 atm
C) 7.3 atm
D) 15 atm
E) none of the above
Correct Answer:
Verified
Q113: At STP,12.69 g of a noble gas
Q114: Water can be formed according to the
Q115: Ammonia gas decomposes according to the equation:
Q116: The vapor pressure of water at 20.0°C
Q117: Which set of conditions reflect STP?
A)298 K,1
Q118: What is the final volume (L)of a
Q119: Suppose a chemical reaction generated a 50%
Q120: What is the final pressure (expressed in
Q121: What is the volume of 28.0 g
Q123: What is the molecular weight of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents