What is the molecular weight of a gas if a 21.0 g sample has a pressure of 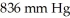 at
at 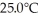 in a
in a 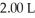 flask?
flask?
(R= 0.0821 L atm/ mol K)
A) 243 amu
B) 1.89 amu
C) 234 amu
D) 11.1 amu
E) none of the above
Correct Answer:
Verified
Q113: At STP,12.69 g of a noble gas
Q114: Water can be formed according to the
Q115: Ammonia gas decomposes according to the equation:
Q116: The vapor pressure of water at 20.0°C
Q117: Which set of conditions reflect STP?
A)298 K,1
Q118: What is the final volume (L)of a
Q119: Suppose a chemical reaction generated a 50%
Q120: What is the final pressure (expressed in
Q121: What is the volume of 28.0 g
Q122: What is the pressure of a 1.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents