Consider the reaction N2(g)+ 3H2(g) 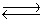 2NH3(g). If nitrogen is removed from the system at equilibrium, what will happen to the hydrogen (H2)concentration?
2NH3(g). If nitrogen is removed from the system at equilibrium, what will happen to the hydrogen (H2)concentration?
Correct Answer:
Verified
Q69: The data below refer to the following
Q70: Ethanol and acetic acid react to form
Q71: Consider the chemical reaction 2NH3(g) 
Q72: The data below refer to the following
Q73: Consider the reaction N2(g)+ 3H2(g) 
Q75: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q77: Consider the chemical reaction 2NH3(g) 
Q78: Consider the reaction N2(g)+ 3H2(g) 
Q79: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q85: What conditions are used in the Haber
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents