Use the given standard enthalpies of formation to calculate ΔH° for the following reaction
3 Fe2O3(s) + CO(g) → 2 Fe3O4(s) + CO2(g) . 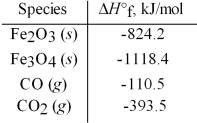
A) -5213.4 kJ
B) -577.2 kJ
C) -47.2 kJ
D) +47.2 kJ
Correct Answer:
Verified
Q28: Water has an unusually high
A)electrical conductivity.
B)heat of
Q49: The heat of combustion per mole for
Q51: Find ΔH for C3H8 (g)+ 6 H2O
Q52: When 0.455 g of anthracene,C14H10,is combusted in
Q53: Find ΔH° for the reaction C3H8(g)+ 5
Q55: The specific heat of copper is 0.385
Q57: 10.0 g of a metal,initially at 25°C,are
Q58: Which is expected to have the lowest
Q59: When 1.50 g of Ba(s)is added to
Q60: For which should the standard heat of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents