Drawings (1) and (2) show the equilibrium vapor pressures of two pure liquids.Which drawing (3) -(6) represents the equilibrium vapor pressure of a solution made by mixing equal moles of each liquid? 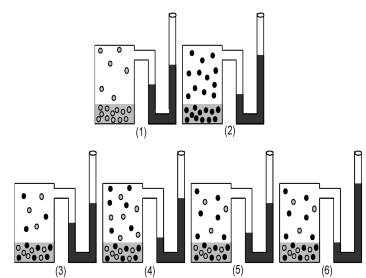
A) drawing (3)
B) drawing (4)
C) drawing (5)
D) drawing (6)
Correct Answer:
Verified
Q84: The following diagram shows a close-up view
Q105: A phase diagram of temperature versus composition
Q106: A phase diagram of temperature versus composition
Q109: A phase diagram of temperature versus composition
Q133: Two beakers,one with pure water (light gray)and
Q134: Drawing (1)shows a system in which an
Q138: At 80°C,pure liquid A has a vapor
Q140: The following diagram shows a close-up view
Q141: What volume of a 0.716 M KBr
Q142: How many grams of KBr are required
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents