The following diagram shows a close-up view of the vapor pressure curves for a pure solvent and a solution containing a nonvolatile solute dissolved in this solvent. 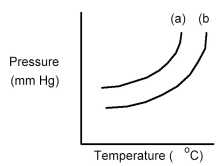
-Which curve is the solvent and what happens to the vapor pressure when the solute is dissolved in the solvent?
A) Curve (a) is the solvent and the vapor pressure decreases.
B) Curve (a) is the solvent and the vapor pressure increases.
C) Curve (b) is the solvent and the vapor pressure decreases.
D) Curve (b) is the solvent and the vapor pressure increases.
Correct Answer:
Verified
Q79: An aqueous CsCl solution is 8.00 wt%
Q80: A solution of 0.2113 g of water
Q81: Drawing (1)shows the equilibrium vapor pressure of
Q82: The following diagram shows a close-up view
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents