Drawing (1) shows a system in which an equilibrium exists between dissolved and undissolved gas particles at P = 1 atm.According to Henry's law,if the pressure is increased to 2 atm and equilibrium is restored,which drawing (2) -(5) best represents the equilibrium at 2 atm? 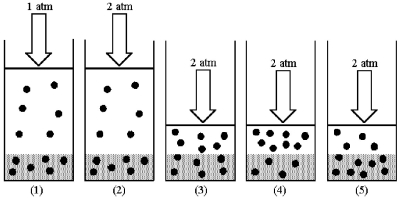
A) drawing (2)
B) drawing (3)
C) drawing (4)
D) drawing (5)
Correct Answer:
Verified
Q84: The following diagram shows a close-up view
Q85: Drawings (1)and (2)show the equilibrium vapor pressures
Q87: Arrows in the energy diagram below represent
Q88: The following diagram shows a close-up view
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents