Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3. 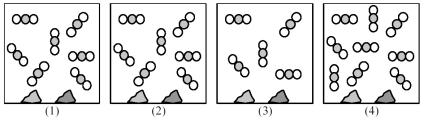
-Which picture (2) -(4) represents the equilibrium mixture after addition of four more CO2 molecules?
A) picture (2)
B) picture (3)
C) picture (4)
D) None of these
Correct Answer:
Verified
Q16: For the reaction: N2(g)+ 2 O2(g)⇌ 2
Q109: Picture (1)represents an equilibrium mixture of solid
Q110: Write the equilibrium equation for the reverse
Q111: Which equilibrium below is homogeneous?
A)CaSO4(s)⇌ Ca2+(aq)+ SO42-(aq)
B)2
Q112: Picture (1)represents an equilibrium mixture of solid
Q113: Write the equilibrium equation for the forward
Q115: Kc is 1.67 × 1020 at 25°C
Q116: If Kc = 0.600,and Kp = 359
Q117: If Kc equals 0.110 at 25°C for
Q119: The decomposition of ammonia is: 2 NH3(g)=
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents