Picture (a) shows p-orbital overlap between carbon and oxygen atoms;picture (b) shows p-orbital overlap between silicon and oxygen atoms. 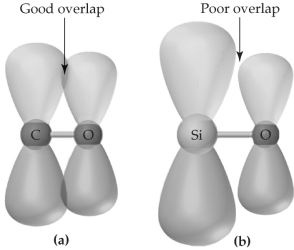
Based on these pictures,it is expected that
A) carbon and oxygen form better σ bonds than silicon and oxygen,so C-O single bonds are stronger than Si-O single bonds.
B) silicon and oxygen form better σ bonds than carbon and oxygen,so Si-O single bonds are stronger than C-O single bonds.
C) carbon and oxygen form better π bonds than silicon and oxygen,so C=O double bonds are stronger than Si=O double bonds.
D) silicon and oxygen form better π bonds than carbon and oxygen,so Si=O double bonds are stronger than C=O double bonds.
Correct Answer:
Verified
Q113: In the following picture of an oxide,darkly-shaded
Q114: In the picture representing binary hydride AHx,lightly-shaded
Q115: Look at the location of elements A,B,C,and
Q117: Look at the location of elements A,B,C,and
Q121: Look at the location of elements A,B,C,and
Q122: The following molecular orbital energy level diagram
Q123: How many liters of hydrogen gas are

