The following molecular orbital energy level diagram shows the energies and occupancies of the MOs derived from the atomic 2p orbitals for an oxygen-containing binary compound of potassium.This compound is a 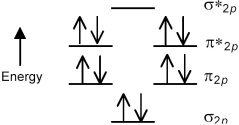
A) peroxide that is attracted by magnetic fields.
B) peroxide that is repelled by magnetic fields.
C) superoxide that is attracted by magnetic fields.
D) superoxide that is repelled by magnetic fields.
Correct Answer:
Verified
Q25: How many mL of O2 gas at
Q117: Look at the location of elements A,B,C,and
Q118: Picture (a)shows p-orbital overlap between carbon and
Q121: Look at the location of elements A,B,C,and
Q123: How many liters of hydrogen gas are
Q124: Look at the location of elements A,B,C,and
Q126: How many grams of water are required
Q127: How many grams of calcium hydride are
Q165: Q167: ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents