Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 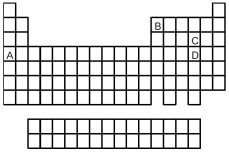
-Which oxide is the most basic?
A) A
B) B
C) C
D) D
Correct Answer:
Verified
Q25: How many mL of O2 gas at
Q121: Look at the location of elements A,B,C,and
Q122: The following molecular orbital energy level diagram
Q123: How many liters of hydrogen gas are
Q126: How many grams of water are required
Q127: How many grams of calcium hydride are
Q128: The oxide of which element,identified by letters
Q129: The following molecular orbital energy level diagram
Q165: Q167: ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents