Multiple Choice
Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 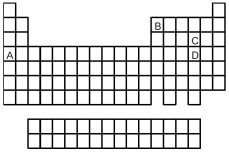
-Which oxide is a solid with an infinitely extended three-dimensional crystal structure?
A) A
B) B
C) C
D) D
Correct Answer:
Verified
Related Questions
Q25: How many mL of O2 gas at
Q117: Look at the location of elements A,B,C,and
Q118: Picture (a)shows p-orbital overlap between carbon and
Q122: The following molecular orbital energy level diagram
Q123: How many liters of hydrogen gas are
Q124: Look at the location of elements A,B,C,and
Q126: How many grams of water are required

