Each of the following containers illustrates a solution in which the black spheres represent solute. 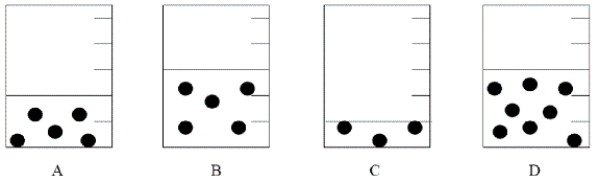 Which is the most concentrated solution?
Which is the most concentrated solution?
A) A
B) All have the same concentration.
C) B
D) C
E) D
Correct Answer:
Verified
Q134: The following change occurs in acidic solution:
_
Q135: What mass of Na2CO3 is present in
Q136: What is the molarity of hydrochloric acid
Q137: A 38.0-g sample of NaOH is dissolved
Q138: In order to prepare a standard 1.00
Q140: In basic solution,H2O2 oxidizes Cr3+ to CrO42-
Q141: The reaction of H2SO4 with NaOH is
Q142: What volume of 1.08 M HCl is
Q143: In order to dilute 71.1 mL of
Q144: Identify the true relationship between molarity and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents