If the average kinetic energy of the molecules of a monatomic ideal gas at 60°C is doubled,what is the resulting temperature of the gas?
A) It becomes 60×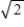 °C.
°C.
B) It becomes 666°C.
C) It becomes 120°C.
D) It becomes 393°C.
Correct Answer:
Verified
Q20: Four moles of nitrogen gas are contained
Q21: A pressure of 2.0 × 10−7 mm
Q22: The average kinetic energy of a molecule
Q23: A quantity of a monatomic ideal gas
Q25: The mass of a hot-air balloon and
Q26: What is the internal energy of 42
Q27: If the temperature of a gas triples,by
Q28: What is the internal energy of a
Q65: The gas,C3H8,is used in outside grills,and several
Q68: The volume holding 0.63 mole of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents