If the temperature of a gas triples,by what factor does the speed of a molecule with the average kinetic energy change?
A) It increases by a factor of 3.
B) It increases by a factor of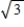 .
.
C) It increases by a factor of 3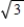 .
.
D) It increases by a factor of 1/3.
Correct Answer:
Verified
Q22: The average kinetic energy of a molecule
Q23: A quantity of a monatomic ideal gas
Q24: If the average kinetic energy of the
Q25: The mass of a hot-air balloon and
Q26: What is the internal energy of 42
Q28: What is the internal energy of a
Q30: A spherical air bubble originating from a
Q31: A tank with a volume of 0.200
Q68: The volume holding 0.63 mole of an
Q84: If the temperature of a volume of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents