What mass of oxygen gas would be consumed by the complete combustion of 7.5 g of a mixture of propane (C3H8) and butane (C4H10) in the mole ratio of 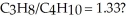
A) 3.9 g
B) 11 g
C) 14 g
D) 21 g
E) 27 g
Correct Answer:
Verified
Q74: What mass of MgCl2 in grams must
Q75: What is the sum of the coefficients
Q76: What mass of water is produced in
Q77: The molarity of a solution that contains
Q78: How much Cl2,in g,is required to produce
Q80: What is the sum of the coefficients
Q81: The chemical reaction during low current discharge
Q82: In the reaction: 4 Zn + K2Cr2O7
Q83: In the following reaction:
2 KClO3(s)→ 2 KCl(s)+
Q84: How many grams of sulfuric acid,H2SO4,can be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents