Write the equilibrium constant expression for the following reaction:
N2(g) + 3 H2(g) ⇌ 2 NH3(g)
A) 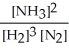
B) 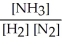
C) 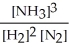
D) 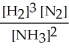
E) 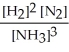
Correct Answer:
Verified
Q55: In a reaction at equilibrium involving only
Q56: Consider the following chemical reaction at equilibrium:
2
Q57: For the reaction below
CO(g)+ 2 H2(g)⇌ CH3OH(g)
The
Q58: Consider the following equation:
N2O4(g)⇌ 2 NO2(g),Kc =
Q59: What is the equilibrium constant expression for:
4
Q61: Write the equilibrium expression Kc for the
Q62: For a reaction,the reaction quotient,Qc > Kc
Q63: For the reaction:
3 Fe(s)+ 4 H2O(g)⇌ Fe3O4(s)+
Q64: For the reaction 2 NO2(g)⇌ N2O4(g)Kp equals
Q65: Two moles of NH3 are initially present
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents