Consider the equilibrium system: N2O4(g) ⇌ 2 NO2(g) for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assuming that the total pressure inside the container is 1.00 atm at equilibrium and that initially only N2O4 was present inside the container,compute 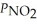
At equilibrium.
A) 0.398 atm
B) 0.113 atm
C) 0.602 atm
D) 0.285 atm
E) 0.715 atm
Correct Answer:
Verified
Q76: Write the equilibrium constant expression for the
Q77: In the reaction:
2 N2O(g)+ N2H4(g)⇌ 3 N2(g)+
Q78: For 2 NO2(g)⇌ N2O4(g),Kc = [N2O4]/[NO2]2.At equilibrium
Q79: Given the following reactions,
2 PCl3(g)⇌ 2 P(g)+
Q80: For the reaction 2 A(g)⇌ B(g)+ C(g),Kc
Q82: Consider the following reaction:
POCl3(g)⇌ POCl(g)+ Cl2(g)with Kc
Q83: A mixture,containing 0.0750 M HCl(g)and 0.0330 M
Q84: A mixture containing 0.392 M A(g)and 0.452
Q85: For the reaction:
PCl3(g)+ Cl2(g)⇌ PCl5(g)at 85 °C,Kp
Q86: Consider the equilibrium system: N2O4(g)⇌ 2 NO2(g)for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents