Calculate Kp for the reaction 2NOCl(g) 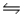 2NO(g) + Cl2(g) at 400°C if Kc at 400°C for this reaction is 2.1 × 10-2.
2NO(g) + Cl2(g) at 400°C if Kc at 400°C for this reaction is 2.1 × 10-2.
A) 2.1 × 10-2
B) 1.7 × 10-3
C) 0.70
D) 1.2
E) 3.8 × 10-4
Correct Answer:
Verified
Q7: The solubility of silver chloride can be
Q9: Calculate Kc for the reaction 2HI(g)
Q10: Consider the two gaseous equilibria: 
Q11: The brown gas NO2 and the colorless
Q13: Which is the correct equilibrium constant expression
Q14: When the following reaction is at equilibrium,
Q15: A reaction with an equilibrium constant Kc
Q15: On analysis, an equilibrium mixture for the
Q16: The equilibrium between carbon dioxide gas and
Q17: When the following reaction is at equilibrium,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents