Kp for the reaction 4CuO(s) 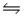 2Cu2O(s) + O2(g) is 0.49 at 1024 °C.Calculate Kc at this temperature
2Cu2O(s) + O2(g) is 0.49 at 1024 °C.Calculate Kc at this temperature
A) 5.8 x 10-3
B) 41
C) 52
D) 4.6 x 10-3
E) 0.49
Correct Answer:
Verified
Q24: Kp for the reaction CO2(g)+ C(s)
Q25: For the nitrogen fixation reaction 3H2(g)+ N2(g)
Q26: At 400ºC, Kc = 64 for the
Q27: At 340 K, Kp = 69 for
Q28: Hydrogen iodide decomposes according to the equation
Q30: At 35ºC, the equilibrium constant for the
Q31: At 700 K, the reaction 2SO2(g)+ O2(g)
Q32: Phosgene, COCl2, a poisonous gas, decomposes according
Q33: Equilibrium is established for the reaction 2X(s)+
Q34: At 250ºC, the equilibrium constant Kp for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents