When the reaction 2H2S(g) 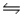 2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.012.Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g) ?
2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.012.Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g) ?
A) 1.06 atm
B) 1.86 atm
C) 0.94 atm
D) 0.90 atm
E) 1.52 atm
Correct Answer:
Verified
Q71: Consider the reaction N2(g)+ 3H2(g) 
Q72: Consider the reaction, N2(g)+ 3H2(g) 
Q73: A solution was prepared such that the
Q74: Given the following data for the reaction:
Q75: Consider the reaction N2(g)+ 3H2(g)
Q77: A quantity of liquid methanol, CH3OH, is
Q78: The data below refer to the following
Q79: When the substances in the equation
Q80: Which of the following rate laws
Q81: Consider the chemical reaction 2NH3(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents