Essay
Consider the reaction N2(g)+ 3H2(g)
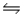
2NH3(g).The production of ammonia is an exothermic reaction.Will heating the equilibrium system increase or decrease the amount of ammonia produced?
Correct Answer:
Verified
The amount...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q70: Consider the reaction N2(g)+ 3H2(g) 
Q71: Consider the reaction N2(g)+ 3H2(g) 
Q72: Consider the reaction, N2(g)+ 3H2(g) 
Q73: A solution was prepared such that the
Q74: Given the following data for the reaction:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents