A quantity of liquid methanol, CH3OH, is introduced into a rigid 3.00-L vessel, the vessel is sealed, and the temperature is raised to 500K.At this temperature, the methanol vaporizes and decomposes according to the reaction CH3OH(g) 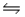
CO(g) + 2 H2(g) , Kc= 6.90×10-2.
If the concentration of H2 in the equilibrium mixture is 0.426M, what mass of methanol was initially introduced into the vessel?
A) 147 g
B) 74.3 g
C) 33.9 g
D) 49.0 g
E) 24.8 g
Correct Answer:
Verified
Q72: Consider the reaction, N2(g)+ 3H2(g) 
Q73: A solution was prepared such that the
Q74: Given the following data for the reaction:
Q75: Consider the reaction N2(g)+ 3H2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents