You take 50 mL of small BB's and combine them with 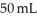 of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
A) Since the density of the small BB's is less than that of the large BB's their volumes do not add directly to one another.
B) This is not possible since the Law of Conservation of Volume would be violated.
C) The total volume actually gets larger since mixing the BB's would leave additional air space because of the difference in size of the two BB sets.
D) Many of the smaller BB's are able to fit within the pockets of space that were empty within the 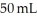 of large BB's.
of large BB's.
Correct Answer:
Verified
Q2: Aristotle described the composition and behavior of
Q4: The same amount of red colored Kool-Aid
Q5: Based on the Law of Mass Conservation,
Q6: Based on experimental evidence, John Dalton postulated
Q6: In what sense can you truthfully say
Q7: Dalton's atomic model gained credibility because _.
A)atoms
Q8: According to Aristotle's hypothesis about matter, wet
Q9: A biological cell is best described as
Q11: Dmitri Mendeleev's chart of elements _.
A)was used
Q16: A TV screen looked at from a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents