Reaction of A (unshaded spheres) with B2 (shaded spheres) is shown schematically in the following diagram.Which equation best describes the stoichiometry of the reaction? 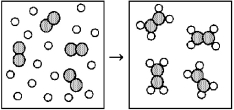
A) 4 A + B2 → 8 A2B
B) 4 A + B2 → A4B2
C) 16 A + 4 B2 → 8 A2B
D) 16 A + 4 B2 → 4 A4B2
Correct Answer:
Verified
Q60: Combustion analysis of a 0.675 g sample
Q61: The following diagram represents the reaction of
Q62: What is the balanced chemical equation for
Q63: Q64: What is the balanced chemical equation for Q66: The following diagram represents the reaction of Q67: What is the stoichiometric coefficient for oxygen Q68: The following diagrams represent the reaction of Q69: The following diagrams represent the reaction of Q70: The following diagram represents the reaction of![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents