The following diagram represents the reaction of A (unshaded spheres) with B (shaded spheres) .What is the balanced chemical equation for this reaction,and what is the limiting reactant? 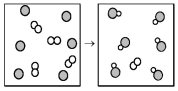
A) A2 + 2B → 2AB;A2 is the limiting reactant.
B) A2 + 2B → 2AB;B is the limiting reactant.
C) 4A2 + 6B → 6AB;A2 is the limiting reactant.
D) 4A2 + 6B → 6AB;B is the limiting reactant.
Correct Answer:
Verified
Q56: Molecular mass can be determined by
A)combustion analysis.
B)mass
Q57: Combustion analysis of 2.796 g of an
Q58: Isoeugenol is the compound which gives the
Q59: Balance the chemical equation given below,and calculate
Q60: Combustion analysis of a 0.675 g sample
Q62: What is the balanced chemical equation for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents