Assume that an aqueous solution of a cation,represented by shaded spheres,is allowed to mix with a solution of an anion,represented by unshaded spheres.Three possible outcomes are represented by boxes (a) -(c) . 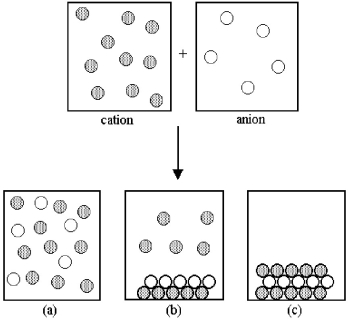
-Which outcome corresponds to the mixing of potassium and sulfide ions shown in the following equation? 2 K+(aq) + S2-(aq) → ?
A) box (a)
B) box (b)
C) box (c)
D) None of these
Correct Answer:
Verified
Q90: Assume that an aqueous solution of hydroxide
Q91: Which outcome corresponds to the mixing of
Q92: Which outcome corresponds to the combination of
Q93: Q94: In an acid-base neutralization reaction 23.74 mL Q96: When 200.mL of 1.50 × 10-4 M Q97: The following pictures represent aqueous solutions of Q98: Which outcome corresponds to the combination of![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents