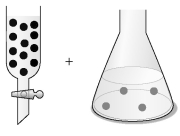
-The concentration of an aqueous solution of NaOCl can be determined by a redox titration with iodide ion in acidic solution: OCl- (aq) + 2 I- (aq) + 2 H⁺ (aq) → Cl⁻ (aq) + I2 (aq) + H2O (l)
Assume that the black spheres in the buret represent I⁻ ions,the gray spheres in the flask represent OCl⁻ ions,the concentration of the I⁻ ions in the buret is 0.120 M,and the volumes in the buret and the flask are identical.What is the concentration of the NaOCl in the flask,and what fraction of the I⁻ solution in the buret must be added to the flask to react with all the OCl⁻ ions?
A) 0.0400 M NaOCl;1/3 of the I⁻ must be added.
B) 0.0400 M NaOCl;2/3 of the I⁻ must be added.
C) 0.0600 M NaOCl;1/3 of the I⁻ must be added.
D) 0.0600 M NaOCl;2/3 of the I⁻ must be added.
Correct Answer:
Verified
Q88: Balance the chemical equation given below,and determine
Q89: Q90: Assume that an aqueous solution of hydroxide Q91: Which outcome corresponds to the mixing of Q92: Which outcome corresponds to the combination of Q94: In an acid-base neutralization reaction 23.74 mL Q95: Assume that an aqueous solution of a Q96: When 200.mL of 1.50 × 10-4 M Q97: The following pictures represent aqueous solutions of Q98: Which outcome corresponds to the combination of![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents