Assume that an aqueous solution of hydroxide ion,OH-,represented by unshaded spheres,is allowed to mix with a solution of an acid,HnA,represented by gray spheres.Three possible outcomes are represented by boxes (a) -(c) ,where the black spheres represent An-,the anion of the acid.For clarity H2O molecules are not shown. 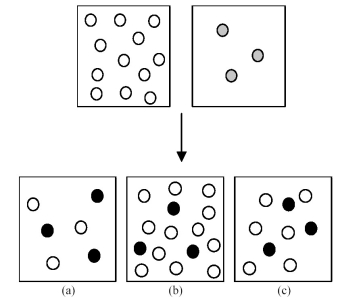
-Which outcome corresponds to the reaction: H3AsO4 + 3 OH- → 3 H2O + AsO43-?
A) box (a)
B) box (b)
C) box (c)
D) None of these
Correct Answer:
Verified
Q85: Assume that an aqueous solution of hydroxide
Q86: Which outcome corresponds to the combination of
Q87: Three different substances,A2X,A2Y,and A2Z,were dissolved in water
Q88: Balance the chemical equation given below,and determine
Q89: Q91: Which outcome corresponds to the mixing of Q92: Which outcome corresponds to the combination of![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents