For acid solutions of the same molarity acid strength is proportional to the equilibrium concentration of H3O+.For equimolar solutions of acids,which equilibrium expression below corresponds to the strongest acid?
A) Kc = 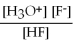
= 3.5 × 10-4
B) Kc = 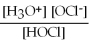
= 3.5 × 10-8
C) Kc = 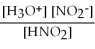
= 4.5 × 10-4
D) Kc = 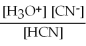
= 4.9 × 10-10
Correct Answer:
Verified
Q38: If Kc = 2.0 × 1033 at
Q39: What is the equilibrium equation for the
Q40: The decomposition of ammonia is: 2 NH3(g)⇌
Q41: When baking soda is heated it decomposes
Q42: At a certain temperature the equilibrium constant,Kc,equals
Q44: Cyclohexane (C6H12)undergoes a molecular rearrangement in the
Q45: Oxalic acid can donate two protons to
Q46: Ammonium carbamate can dissociate into gases at
Q47: Gaseous hydrogen bromide decomposes at elevated temperatures
Q48: Salt solubilities can be compared by the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents