Gaseous hydrogen bromide decomposes at elevated temperatures according to the following equation: 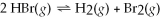 . At a certain temperature a 2.00 L flask is initially filled only with 0.600 mol of HBr.What is the value of Kc at that temperature if the flask contains 0.104 mol of H2 at equilibrium?
. At a certain temperature a 2.00 L flask is initially filled only with 0.600 mol of HBr.What is the value of Kc at that temperature if the flask contains 0.104 mol of H2 at equilibrium?
A) 7.04 × 10-2
B) 4.40 × 10-2
C) 3.00 × 10-2
D) 2.10 × 10-1
Correct Answer:
Verified
Q42: At a certain temperature the equilibrium constant,Kc,equals
Q43: For acid solutions of the same molarity
Q44: Cyclohexane (C6H12)undergoes a molecular rearrangement in the
Q45: Oxalic acid can donate two protons to
Q46: Ammonium carbamate can dissociate into gases at
Q48: Salt solubilities can be compared by the
Q49: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q50: The equilibrium constant Kc for the reaction
Q51: Consider the reaction HCO3- (aq)+ H2O (l)⇌
Q52: An equilibrium mixture of CO,O2 and CO2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents