Water can be made from elemental hydrogen and oxygen.Which equation can be used to determine ΔGform from its elemental parts?
A) ΔGform = Δ 
- Δ
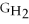
- Δ
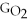
B) ΔGform = 2Δ 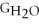
- 2Δ
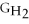
- Δ
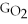
C) ΔGform = Δ 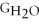
- 2ΔGH - ΔGO
D) ΔGform = 2Δ 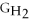
+ Δ
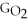
- 2Δ
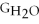
Correct Answer:
Verified
Q54: A positive value of ΔG°f for a
Q55: Calculate the standard free energy change at
Q56: At 2600 K,ΔG° = 775 kJ for
Q57: A reaction has ΔH° = -60.9 kJ/mol
Q58: Which is the lowest at 25°C?
A)ΔG°f for
Q60: Which equation can be used to determine
Q61: If ΔG° is negative for a reaction,
A)K
Q62: An ideal gas is expanded at constant
Q63: What is the relationship between ΔG and
Q64: ΔG = ΔG° for a reaction
A)if Q
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents