Calculate the standard free energy change at 25°C for the reaction 2 NO(g) + O2(g) → 2 NO2(g) . 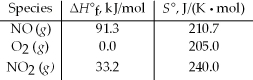
A) -4.7 kJ
B) -72.6 kJ
C) -157.8 kJ
D) -532.6 kJ
Correct Answer:
Verified
Q50: For any thermodynamic function Y,ΔY° for a
Q51: The signs of ΔG,ΔH,and ΔS at 25°C
Q52: A reaction has ΔH° = 61.9 kJ/mol
Q53: At 25°C,ΔG° = -198 kJ for the
Q54: A positive value of ΔG°f for a
Q56: At 2600 K,ΔG° = 775 kJ for
Q57: A reaction has ΔH° = -60.9 kJ/mol
Q58: Which is the lowest at 25°C?
A)ΔG°f for
Q59: Water can be made from elemental hydrogen
Q60: Which equation can be used to determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents