Which equation can be used to determine ΔGform for C2H5OH from its elemental parts.Be sure that the reaction is balanced. 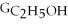
A) ΔGform = Δ 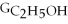
- ΔGC - Δ
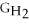
- Δ
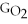
B) ΔGform = 2Δ 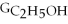
- 4ΔGC - 6Δ
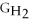
- Δ
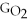
C) ΔGform = 2ΔGC + 6ΔGH + ΔGO
D) ΔGform = Δ 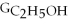
- 2ΔGC - 3Δ
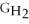
- Δ
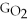
Correct Answer:
Verified
Q55: Calculate the standard free energy change at
Q56: At 2600 K,ΔG° = 775 kJ for
Q57: A reaction has ΔH° = -60.9 kJ/mol
Q58: Which is the lowest at 25°C?
A)ΔG°f for
Q59: Water can be made from elemental hydrogen
Q61: If ΔG° is negative for a reaction,
A)K
Q62: An ideal gas is expanded at constant
Q63: What is the relationship between ΔG and
Q64: ΔG = ΔG° for a reaction
A)if Q
Q65: If ΔG is small and positive,
A)the forward
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents