Consider the following gas-phase reaction of A2 (shaded spheres) and B2 (unshaded spheres) :
A2(g) + B2(g) ⇌ 2 AB(g) ΔG ° = +25 kJ 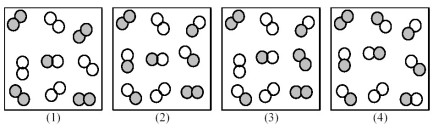
-Which of the above reaction mixtures has the most spontaneous forward reaction?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:
Verified
Q95: Consider the following gas-phase reaction of A2
Q96: Consider the reaction 2A(g)⇌ A2(g).The following pictures
Q97: Q98: The chemical system shown below is at Q99: Consider the reaction 2A(g)⇌ A2(g).The following pictures Q101: For a particular process,ΔG = ΔH at Q102: Which of the following straight chain molecules Q103: Predict the sign of ΔS for each Q104: Which has the highest standard molar entropy Q105: Which has the lowest entropy?![]()
A)CH3OH(s,-26°C)
B)CH3OH(s,-13°C)
C)CH3OH(l,16°C)
D)CH3OH(l,30°C)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents