Multiple Choice
Consider the following gas-phase reaction of A2 (shaded spheres) and B2 (unshaded spheres) :
A2(g) + B2(g) ⇌ 2 AB(g) ΔG ° = +25 kJ 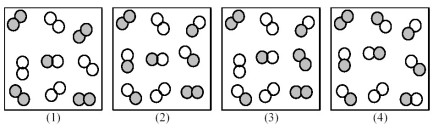
-Which of the above reaction mixtures is ΔG of reaction = ΔG °?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:
Verified
Related Questions
Q90: For which process is the sign of
Q91: Consider the reaction 2A(g)⇌ A2(g).The following pictures
Q92: The figure below represents the spontaneous reaction
Q93: Q94: Predict the sign of ΔS of the Q96: Consider the reaction 2A(g)⇌ A2(g).The following pictures![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents