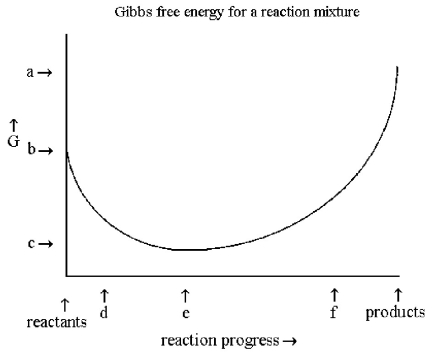
-According to the diagram above,
A) ΔG° is positive and the equilibrium composition is rich in products.
B) ΔG° is positive and the equilibrium composition is rich in reactants.
C) ΔG° is negative and the equilibrium composition is rich in products.
D) ΔG° is negative and the equilibrium composition is rich is reactants.
Correct Answer:
Verified
Q92: The figure below represents the spontaneous reaction
Q93: Q94: Predict the sign of ΔS of the Q95: Consider the following gas-phase reaction of A2 Q96: Consider the reaction 2A(g)⇌ A2(g).The following pictures Q98: The chemical system shown below is at Q99: Consider the reaction 2A(g)⇌ A2(g).The following pictures Q100: Consider the following gas-phase reaction of A2 Q101: For a particular process,ΔG = ΔH at Q102: Which of the following straight chain molecules![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents