Calculate the equilibrium constant for the reaction below if a tank was found to contain 0.106 M O2,0.00652 M SO3 and 0.00129 M SO2.
2 SO3 (g) 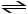
2 SO2 (g) + O2 (g)
A) 6.78 × 10-2
B) 1.34 × 10-2
C) 4.15 × 10-3
D) 4.35 × 10-2
Correct Answer:
Verified
Q63: Match the following.
-A process or reaction that
Q64: If we add a catalyst to the
Q65: Diatomic nitrogen is added to the equilibrium
Q66: Match the following.
-The amount of energy which
Q68: Match the following.
-A process or reaction which
Q69: Match the following.
-A process or reaction which
Q70: Which change to this reaction system would
Q71: The position of the equilibrium for a
Q72: Match the following.
-A state in which the
Q73: 2 SO2 (g)+ O2 (g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents