2 SO2 (g) + O2 (g) 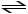
2 SO3 (g) + heat K = 4.8 × 1027
Which statement about this system is not true?
A) At equilibrium SO3 is the predominant substance.
B) Heating the system will cause breakdown of SO3.
C) Adding SO2 will cause an increase in the amount of SO3.
D) Removing O2 will cause an increase in the amount of SO3.
E) The large value of K means that the reaction essentially goes to completion.
Correct Answer:
Verified
Q63: Match the following.
-A process or reaction that
Q64: If we add a catalyst to the
Q65: Diatomic nitrogen is added to the equilibrium
Q66: Match the following.
-The amount of energy which
Q68: Match the following.
-A process or reaction which
Q69: Match the following.
-A process or reaction which
Q70: Which change to this reaction system would
Q71: Calculate the equilibrium constant for the reaction
Q71: The position of the equilibrium for a
Q72: Match the following.
-A state in which the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents