For the equilibrium N2O4(g) 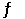 2NO2(g),plot,on the same graph,the forward and reverse reaction rates as a function of time.If possible,mark on the graph where equilibrium is reached.
2NO2(g),plot,on the same graph,the forward and reverse reaction rates as a function of time.If possible,mark on the graph where equilibrium is reached.
Correct Answer:
Verified
Q5: What is the relation between K and
Q6: For the equilibrium CaCO3(s) Q7: If Q7: Consider the reaction Q10: Consider the reaction Q10: Consider the following reaction at a certain Q11: Consider the reaction Q11: Consider the reaction NO(g)+ Q84: If a 1-L flask containing D2(g),N2(g),and ND3(g)at Q110: Calculate ![]()
CuSO4(s)
CuSO4(s)
2CuBr2(s) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents