For the equilibrium CaCO3(s) 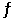 CaO(s)+ CO2(g),(a)represents the composition at equilibrium at a certain temperature.In (b),a small amount of Ca*CO3(s)has been added (Ca*CO3(s)represents Ca14CO3(s)or labeled calcium carbonate).Draw the composition in (c)at equilibrium and explain your drawing.
CaO(s)+ CO2(g),(a)represents the composition at equilibrium at a certain temperature.In (b),a small amount of Ca*CO3(s)has been added (Ca*CO3(s)represents Ca14CO3(s)or labeled calcium carbonate).Draw the composition in (c)at equilibrium and explain your drawing. 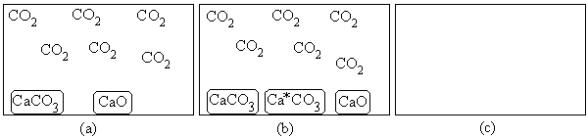
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: For the equilibrium N2O4(g) Q5: What is the relation between K and Q7: If Q7: Consider the reaction Q10: Consider the reaction Q10: Consider the following reaction at a certain Q11: Consider the reaction Q11: Consider the reaction NO(g)+ Q84: If a 1-L flask containing D2(g),N2(g),and ND3(g)at Q110: Calculate ![]()
CuSO4(s)
CuSO4(s)
2CuBr2(s) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents