Use the following to answer questions 55-58: 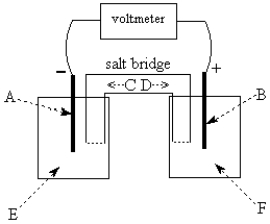
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or zinc.
Correct Answer:
Verified
Q243: Consider the following cell:
Zn(s)|Zn2+(aq,0.200 M)M H+(aq,?)|H2(g,1.00
Q244: Use the following diagram of a cell
Q245: Use the following to answer questions 55-58:
Q246: Use the following diagram of a cell
Q247: Use the following diagram of a
Q249: If 8686 C of charge is passed
Q250: The products of the electrolysis of CuSO4(aq)
Q251: Use the following to answer questions 55-58:
Q252: How long will it take to deposit
Q253: Of the following metals, which metal would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents