Use the following diagram of a cell to answer questions 59-64: 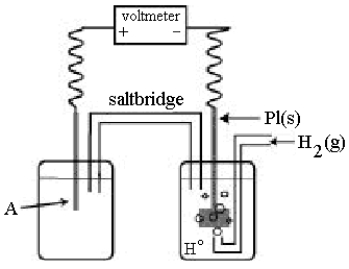
-In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,
Which electrode is negative?
Correct Answer:
Verified
Q241: How many moles of O2(g) are produced
Q242: The half-reaction that occurs at cathode when
Q243: Consider the following cell:
Zn(s)|Zn2+(aq,0.200 M)M H+(aq,?)|H2(g,1.00
Q244: Use the following diagram of a cell
Q245: Use the following to answer questions 55-58:
Q247: Use the following diagram of a
Q248: Use the following to answer questions 55-58:
Q249: If 8686 C of charge is passed
Q250: The products of the electrolysis of CuSO4(aq)
Q251: Use the following to answer questions 55-58:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents