The rate of disappearance of HBr in the gas phase reaction 2HBr (g) → H2 (g) + Br2 (g)
Is 0.190 M 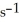 at 150 °C.The rate of appearance of Br2 is ________ M
at 150 °C.The rate of appearance of Br2 is ________ M 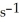 .
.
A) 2.63
B) 0.095
C) 0.0361
D) 0.380
E) 0.436
Correct Answer:
Verified
Q84: The reaction A → B is first
Q85: The isomerization of methylisonitrile to acetonitrile CH3NC
Q86: The rate constant for a particular zero-order
Q87: Nitrogen dioxide decomposes to nitric oxide and
Q88: At elevated temperatures,dinitrogen pentoxide decomposes to nitrogen
Q90: What value is represented by the y-intercept
Q91: What is the rate constant (
Q92: What is the half-life of an unknown
Q93: At elevated temperatures,methylisonitrile (CH3NC)isomerizes to acetonitrile (CH3CN):
Q94: The reaction A → B is first
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents